Background
Despite international unrelated donor (URD) registries listing >36 million volunteer donors, 25-80% of U.S. patients lack an HLA-matched (8/8 alleles) URD, with greater disparities in access for ethnic minorities. We hypothesized that hematopoietic cell transplantation (HCT) with a mismatched unrelated donor (MMUD) using post-transplant cyclophosphamide (PTCY), a strategy successful in overcoming genetic disparity in related haplo-identical donor HCT (haplo HCT) would be associated with similar outcomes to haplo HCT and expand access to HCT. Selecting a MMUD may have benefits over a haplo donor, such as removing risk associated with donor specific antibodies, and allowing for preferential selection of other favorable donor factors, such as younger age.
Methods
The NMDP/BTM performed a prospective phase II study of MMUD bone marrow (BM) HCT with PTCY for patients with hematologic malignancies, with the primary hypothesis that 1-year overall survival (OS) would be 65% or greater. Patients with a suitable HLA matched sibling or URD were excluded. Matching for 4-7/8 at HLA-A, -B, -C, and -DRB1 was permitted. 80 patients enrolled (40 myeloablative conditioning (MAC) - using cyclophosphamide/TBI (n=6), busulfan/cyclophosphamide (n=3) or fludarabine/ busulfan (n=31); 40 reduced intensity conditioning (RIC) - using fludarabine/cyclophosphamide/TBI) at 11 U.S. transplant centers between Dec 2016 and March 2019. Patients received a fresh BM graft, PTCY on days +3, +4, with sirolimus/mycophenolate mofetil starting on day +5. Regimen intensity was at the center's discretion. We compared outcomes to three groups of contemporaneous controls, all receiving PTCY, whose data was collected by CIBMTR: MMUD using peripheral blood stem cells (PBSC) (n=143), and haplo using BM (n=398) or PBSC (n=1191). All analyses used standard stepwise Cox regression modeling, performed separately for MAC and RIC cohorts. The main variable of interest was the donor/graft type (all groups compared to the trial cohort of MMUD BM). Statistical significance is p=0.05.
Results
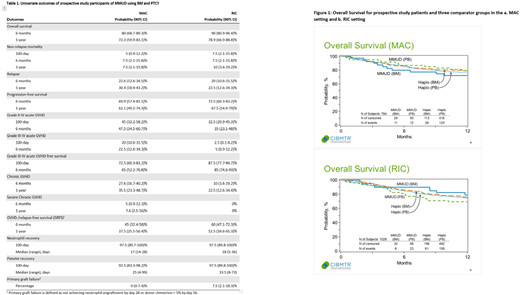
The study achieved its primary endpoint with 1-year OS of 76% (90% CI: 67.3-83.3) in the entire cohort; survival and GVHD-/relapse-free survival (GRFS, defined in table 1) were 72% and 37% in the MAC cohort and 79% and 53% in the RIC cohort, respectively. Additional study outcomes are shown in Table 1. Median patient age was 49 years old (yo) (range: 18-66, MAC) and 60 yo (range: 23-70, RIC). HCT were performed for acute leukemia (n=58), MDS (n=2), lymphoma (n=17) and CLL (n=3). Disease risk index was low (n=11), intermediate (n=63), high (n=13) or very high (n=4). KPS was <90 and HCT-CI was ≥3 in 43% and 54% of patients, respectively. Notably, 48% of patients were ethnic minorities and 39% were <7/8 HLA matched with their donor. 59% of donors were <30 yo and 55% ABO matched with the recipient. Multivariate analysis comparing the study population to the comparator groups found no significant differences in OS (figure 1), GRFS, PFS, NRM or II-IV grade aGVHD in either the MAC or RIC setting, and no differences in III-IV aGVHD or relapse in the RIC setting. In the MAC setting, there was significantly lower grade III-IV aGVHD in haplo BM (HR 0.12, p=0.001), haplo PBSC (HR 0.45, p=0.026), with a trend in MMUD PBSC (HR 0.37, p=0.078), compared to the study subjects. Relapse risk was lower in haplo BM (HR 0.44, p=0.023) and haplo PBSC (HR 0.42, p=0.005), but not significantly different to MMUD PBSC (HR 0.52, p=0.119) when compared to study subjects. Additionally, younger donor age was associated with lower NRM and less aGVHD in the MAC setting and better GRFS, DFS, lower grade III-IV aGVHD, cGVHD and NRM in the RIC setting.
Conclusion
Our prospective study demonstrated that outcomes using MMUD in the setting of PTCY are similar to those obtained using a haplo donor. Approximately half of the study participants were ethnic minorities, a figure consistent with expectations based on donor availability within registries, but exceeding expectations in accrual to a prospective study. These results support the feasibility of using volunteer MMUDs to expand access to HCT, especially for ethnically diverse patients. Next steps include expanding the MMUD PTCY approach to incorporate PBSC as a graft source and prospectively studying questions related to selection based on donor characteristics and additional GVHD prophylaxis agents.
Shaw:Orca Bio: Consultancy. Khimani:Bristol Myers Squibb-Moffitt-Alliance: Research Funding. Shah:Cell Vault: Research Funding; Celgene: Consultancy, Honoraria; Verastim: Consultancy; Lily: Consultancy, Honoraria; TG Therapeutics: Consultancy; Incyte: Consultancy; Miltenyi Biotec: Honoraria, Research Funding; Kite Pharma: Consultancy, Honoraria. Farhadfar:CSL Behring: Research Funding; Incyte pharmaceutical: Other: Member of GVHD advisory forum. Hardy:Kite/Gilead: Other: Advisory Board Member; Incyte Corporation: Other: Advisory Board Member; American Gene Technologies: Other: DSMB Member. Perales:Medigene: Membership on an entity's Board of Directors or advisory committees, Other; NexImmune: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees, Other; Bellicum: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Nektar Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria; Kite/Gilead: Honoraria, Research Funding; Miltenyi Biotec: Research Funding; Incyte Corporation: Honoraria, Research Funding; MolMed: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Omeros: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cidara Therapeutics: Other; Celgene: Honoraria. Komanduri:Kite/Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Adaptimmune: Membership on an entity's Board of Directors or advisory committees; Atara: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Takeda: Consultancy; Kiadis: Consultancy. Luznik:Merck: Research Funding, Speakers Bureau; AbbVie: Consultancy; WindMil Therapeutics: Patents & Royalties: Patent holder; Genentech: Research Funding. Pidala:Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Takeda: Research Funding; Janssen: Research Funding; Johnson and Johnson: Research Funding; Pharmacyclics: Research Funding; Abbvie: Research Funding; BMS: Research Funding. Devine:Magenta Therapeutics: Consultancy. Bolanos-Meade:Incyte: Other: DSMB Fees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal